| [Site Map][Japanese][English] |
-
Student Recruitment
-
Project
- Computational Science
- Nonequilibrium Systems
- Chemical Reactions
- Macroscopic Chemical Phenomena
- Life Phenomena
- Multiscale Information Processing
- Industry Applications
- Meetings
- FY 2022
- FY 2021
- FY 2020
- FY 2019
- FY 2018
- FY 2017
- FY 2016
- FY 2015
- FY 2014
- FY 2013
- FY 2012
- FY 2011
- FY 2010
- FY 2009
- FY 2008
- FY 2007
- FY 2006
- FY 2005
- FY 2004
- FY 2003
- FY 2000
- FY 2019
- FY 2018
- FY 2017
- FY 2016
- FY 2015
- FY 2014
- FY 2013
- FY 2012
- FY 2011
- FY 2010
- FY 2009
- FY 2008
- FY 2007
- FY 2004-2006
- FY 2003
ConceptResearchesMeetings & Seminars
Open Seminars
References
Facilities
-
People
- Staff
- PostDocs & Students
- OB & OG
- OB & OG Messages
- FY 2018-
- FY 2015-2017
- FY 1999-2014
MembersSnapshots -
Access
-
Link
Application to Industrial materials and devices by employing Computational Molecular Technology
Today, a variety of methods of computational science based on the quantum chemistry and molecular simulation such as molecular dynamics (MD) and Monte Carlo (MC) method, have been greatly advanced. In fact, it has become a common practice to apply those methods along with experimental ones to solve existing problems. It is highly anticipated that the theoretical technique at both atomic and molecular level using high-performance computer simulation, i.e., Computational Molecular Technology, will help us understand the phenomena on the time and space scale that are hardly been observed by experimental methods. Thus, it has been applied to several complex systems, namely chemically reacting systems in condensed phase and biomolecular systems, and their coupling complex systems and non-equilibrium systems, along with some materials and devices with high industrial values. This page will show you some of the examples which are actively studied on in our laboratory.
Reverse Osmosis Membrane
Currently, some kinds of macromolecular material are industrially produced and used in a wide range of applications in daily life. As one example, polymer membrane, i.e., the FT-30 is well-known as a useful aromatic polyamide thin-film for reverse osmosis (RO) technique. This membrane is synthesized by the polycondensation at the interface between the aqueous and organic phases, at which two kinds of monomer molecules dissolve mutually in other phase. To improve the performance for RO membrane, it is required that its saltwater flux is maximized while keeping higher efficiency of the salt ion rejection performance. Until now, it has been studied by various experimental methods to improve the performance of the FT-30 membrane. However, more detailed microscopic special arrangement of the membrane has not been characterized yet. Therefore, we made all-atom membrane models from constituent of monomers, simulating a series of polymerization reactions with the hybrid MC/MD reaction method. By comparing our calculated results with the corresponding experimental ones, we have been investigating the validity of the membrane model. Additionally, we aim to clarify the physicochemical mechanism of water permeation and salt rejection through the membrane. The goal of our study is to propose a new material design of RO membrane with higher performance based on computational simulation and analysis.
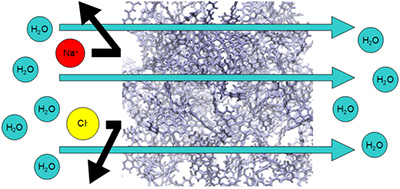
Figure 1. Schematic image of water-flow mechanism
[Reference] Y. Suzuki, Y. Koyano, M. Nagaoka, J. Phys. Chem. B, 119(22), 6776-6785 (2015).
Sodium-ion Battery
Recently, Lithium-ion batteries play a vital role in our daily life especially in currently industries as they are the prominent power source for cell phones, laptop computers, digital cameras, power tools, electrical assist bicycles, and many consumer products. However, those secondary batteries consist of the lithium (Li) element which is a rare metal, and thus there is a concern for the sustainable supply of those batteries. Therefore, the secondary battery based on sodium-ion (Na+) is getting more attention as a replacement of lithium-ion now since supplying sodium is easier than lithium. However, to develop the Na+ battery to the practical use level at which it functions enough to match the performance of the Li+ battery, it is necessary to re-design the materials (such as an electrode, an electrolyte, the additive, and so on). One of the problems on the practical application is that SEI film doesn't form in the same materials as the lithium-ion's battery. The SEI film is a passivity state film which is formed first charge process on the negative electrode and Na+ battery can perform as a secondary one with more stable charge (and discharge), by forming this film. From a recent experimental study, it is found that SEI film can be formed when proper materials are selected. But, it is very difficult for researchers to observe the SEI form process in experiment and the SEI formation process has microscopically been not clear yet. Thus, in our laboratory, we aim to elucidate the SEI film formation process with all-atom molecular simulation and contribute to development of innovative Na+ battery.
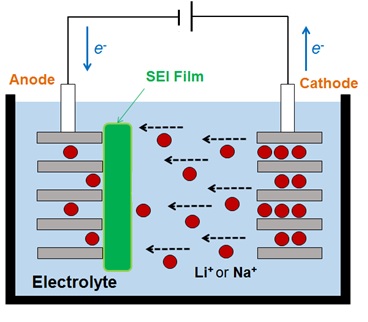
Figure 2. Schematic image of charge and dis-charge cycle in secondary battery
[Reference]
N. Takenaka, Y. Suzuki, H. Sakai, M. Nagaoka, J. Phys. Chem. C, 118(20), 10874-10882 (2014).
N. Takenaka, H. Sakai, Y. Suzuki, P. Uppula, M. Nagaoka, J. Phys. Chem. C, 119(32), 18046-18055 (2015).
macromolecular material
高分子とは、分子量が約1万以上である化合物の総称。例えば、プラスティックやナイロン、ポリエステル、ゴムなどが挙げられる。
reverse osmosis (RO) technique
海水や汽水から淡水を造り出すための技術。薄膜を通して不純物である塩をろ過し、水のみを獲得する方法。
FT-30
m-phenylenediamine(MPD)とbenzene 1,3,5-tricarboxylic acid chloride(TMC)の二つの単量体分子から形成される芳香族ポリアミド膜。逆浸透を利用した淡水化技術に対して高い性能を有し、逆浸透膜として代表されるポリマー膜。
polycondensation
縮合重合とも言う。同種または異種の2分子が特定の分子を分離・結合し(縮合)、それが繰り返し生じることで分子量がより大きい化合物が生成される反応(重合)のこと。
secondary battery
充電することにより繰り返し利用可能な電池のこと。ナトリウムイオン電池では、充電時に正極からナトリウムイオンが電解液に抜け出し、負極に貯蔵される。放電時には、負極に蓄えられたナトリウムイオンが正極に移動することにより、外部回路に電流が流れる。
rare metal
非鉄金属のうち、産出量が少なく、産業界での流通量が少ない金属のこと。
electrode
電池の充電・放電のために、外部と電気的に接続された部分。一般的なリチウムイオン二次電池で用いられているグラファイトを負極に用いても、ナトリウムイオン二次電池は正常に動作しない。そのため、正極にはNa由来酸化物(例えばNaFeO2,NaCrO2)、負極にはハードカーボンを使用することが提案されている。
electrolyte
別名、電解質溶液ともいう。イオン性物質を溶解させて作られる電気伝導性を有する溶液のこと。
additive
電解液に加えることにより、二次電池の性能(寿命など)を改善することが可能な物質のこと。例えば、リチウムイオン電池の電解液にビニレンカーボネートを加えると、電極に安定なSEI膜が形成されて電池がより長寿命になることが知られている。
SEI film
固体電解質相間(Solid Electrolyte Interphase, SEI)膜の略。イオン伝導性を持つ一方で電子伝導性を持たない薄膜。ゆえに負極―電解液間でナトリウムイオンの挿入脱離を許容するが、電極表面の電解液の還元分解は抑制される。
passivity state film
電極表面において形成され、電極―電解液間の化学反応を阻害する薄膜のこと。