From elementary reaction to complex chemical reaction
From a microscopic view, various natural phenomena involving a chemical reaction are generally caused by a series of elementary step continuously occurring at several points in phase space. When paying attention to each elementary step, it could be expressed as transitions between two regions in phase space. Since reaction system in most cases make a long stay within a stable region, regardless of the speed of its transition (~ a few ps), that step itself must be a 'rare event' happening extremely rarely. In addition, dynamics and static properties of stereo-specificity and aggregated structure are induced by cumulative results of an enormous number of elementary step in complex chemical reactions occurring in a long time scale (>=μs). It is, therefore, absolutely impossible to directly simulate them by employing traditional molecular simulation methods Under the circumstances, in our laboratory, we have proposed and developed Red Moon method (the hybrid Monte Carlo (MC) / Molecular Dynamics (MD) reaction method) to realize the practical atomistic simulation for massive complex chemical reaction systems.
Red Moon Method (The Hybrid MC/MD Reaction Method)
Red Moon method (the hybrid MC/MD reaction method) is a practical 'atomistic' molecular simulation for the complex chemical reaction systems. In this method, Monte Carlo (MC) method is applied to drive such chemical reactions, etc., that are rare events, while the molecular motions (molecular translation, rotation and vibration) are directly treated by using MD. Namely, by stochastically promoting the various chemical reactions, etc., this approach can handle the chemical phenomena appearing with the time and size (>100 A and μs ~ ms) which cannot be realized in conventional ones. In fact, the system can reach a state of chemical equilibrium (or stationary state) due to the above driving mechanism which generates such rare events as chemical reactions within the practical computational time (Rare event-driving mechanism). The concept of Red Moon method (the hybrid MC/MD reaction method) is shown in Figure 1.
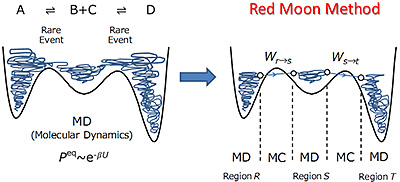
Figure 1. The concept of Red Moon method (the hybrid reaction method).
First, we execute MD simulation in the region R until (one or) some pairs of atoms meet the necessary conditions and select configuration state r. Next, one of the pairs is virtually reacted to generate a configuration state s, relaxing the whole system through short MD simulation. In this step, after computing the potential energy difference ΔUrs (= Us - Ur) between the reactant and product, the present reaction event is accepted (or rejected) according to the transition probability under the Metropolis scheme,
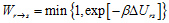
By repeating such a combined cycle (MC/MD cycle) consisting of a couple of MC and MD treatment, the present method can promote changes of state that is brought about by a chemical reaction, and achieve a long-time simulation of large-scale chemical reaction systems.
We call a group of hybrid MC/MD methods of this kind, Red Moon method, from the acronyms of "Rare event-driving methodology of necessity". We would like you to refer to the paper listed below to learn more about our reasoning for the naming.
[References] 1. M. Nagaoka, Y. Suzuki, T. Okamoto, N. Takenaka, Chem. Phys. Lett., 583, 80-86 (2013).
2. Y. Suzuki, M. Nagaoka, J. Chem. Phys., 146, 204102 (2017).
2-Chlorobutane racemization in DMF solution
In the field of stereochemistry, it is known that 2-chlorobutane molecule is one of typical organic compounds having one chiral carbon and exists as two stereoisomers, i.e., (R)- and (S)-enantiomers. In an equilibrium state, 2-chlorobutane molecules in the organic solution constantly isomerize between the two enantiomers and its enantiomeric excess is almost zero (~0% e.e.), which means they are 50:50 mixture of (R)- and (S)-enantiomers (racemic mixture).
(red, (R)-2-chlorobutane; blue, (S)-2-chlorobutane; black, 2-butylcation and chloride ion; sphere-stick model, DMF).
We applied Red Moon (the hybrid MC/MD reaction) method to 2-chlorobutane racemization in N,N-dimethylformamide (DMF) solution, and performed at the initial optical pure state full of (R)-2-chlorobutane molecules (100% e.e.). The simulation results are presented by movie in Figure 2, where the changes of mixed state and numbers of (R)- and (S)-2-chlorobutane molecules and 2-butylcations and chloride ion are shown as a function of the MC/MD cycle. It was observed that decomposition of 2-cholorobutane and their recombination occurred frequently and the numbers of their enantiomers changed during Red Moon simulation. Eventually, the whole system reached an equilibrium state with the number of molecules thermally fluctuating and two enantiomers existing in equal numbers of ~25. In other words, application of Red Moon method to 2-chlorobutane racemization in DMF solution starting in the optical pure state (100% e.e.) was found to successfully provide such an atomistic state with 0% e.e.; the expected purity of (R)- to (S)-enantiomers of the racemic mixture in chemical equilibrium.
[References] 1. M. Nagaoka, Y. Suzuki, T. Okamoto, N. Takenaka, Chem. Phys. Lett., 583, 80-86 (2013).
phase space
粒子の位置と運動量を変数とする座標軸によって規定される分子の運動を記述するための抽象的な次元空間。
Monte Carlo
ある与えられた集合の特性を表すパラメータの値を求めたいとき、乱数を使ってその集合から要素を選び出し(サンプリングするという)、そのサンプル集団を統計処理することによりパラメータの近似値を計算する数値的手法。
equilibrium state
外界の条件が一定の時、系が最終的に到達する最も安定な状態。
stationary state
時間変化のない平衡状態とは異なり、時間の原点をずらしても変化しない状態。例えば、流速が一定の系の状態。定常な確率過程 x(t) について、その相関関数 R(t1, t2) = < x(t1) x(t2) >は、τ = t1 - t2 の関数となり、R(t1, t2) = R(τ) = R(-τ) を満たす。
Metropolis scheme
乱数を使って次々と新しい状態を生成してゆく過程を繰り返し、その系列の十分後の方では各状態がボルツマン分布則に従うことを保証するアルゴリズム。
chiral carbon
4種の異なる原子または基と結合した炭素原子。
stereoisomers
構造式は同じだが、立体的に重ね合わせることのできない分子。
racemic mixture
系内の二つの鏡像体が等量存在する状態のこと。
enantiomeric excess
光学純度を表し、不斉化合物の鏡像体が混合する割合e.e.(enantiomeric excess)で表される。e.e.は鏡像体の多い方の物質量から少ない方の物質量を引き、全体の物質量で割った値で表される。
racemization
純粋な光学活性分子が環境要因(熱や光、あるいは酸・アルカリなど)によって異性化反応を生じ、経時変化に伴って二つの鏡像体の等量混合物になること。